Progress in Drug Delivery Systems
Innovations in Medication Formulation and Administration
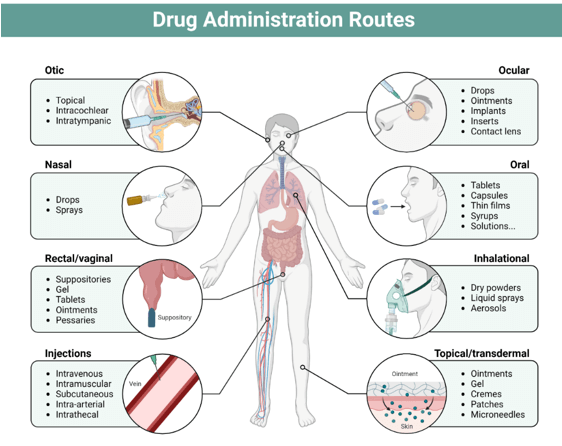
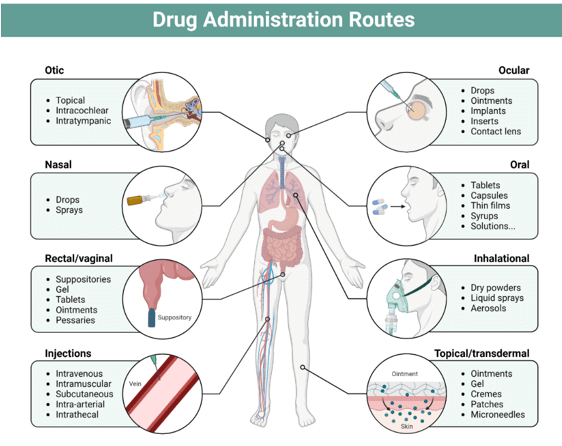
Drug delivery systems have transformed how therapeutic molecules are developed and stored. These technologies ensure that medications reach their target areas within the body, enhancing their effectiveness and reducing unnecessary accumulation. The methods of administration are varied, including oral, buccal, sublingual, nasal, ocular, transdermal, subcutaneous, rectal, vaginal, and intravesical routes.
The Importance of Controlled Drug Release
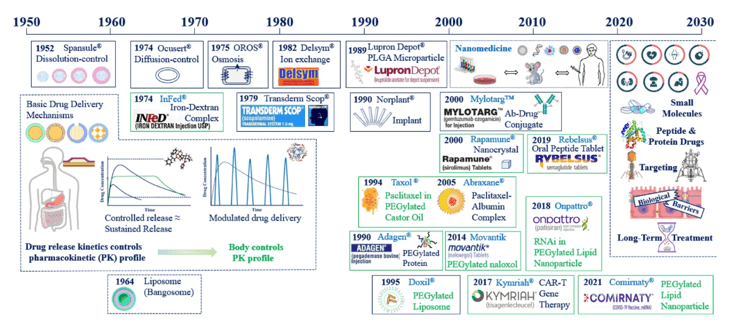
In recent decades, these systems have significantly enhanced disease treatment by improving systemic circulation and better managing pharmacological effects. The concept of controlled drug release, established in the 1950s, has shown significant advantages over conventional medications. By releasing drugs at a predetermined rate over a specific period, it ensures they are not compromised by the body’s physiological conditions, which can extend their effectiveness from days to even years.
Benefits of Advanced Drug Delivery Systems
Controlled drug release systems enhance solubility, target site accumulation, efficacy, pharmacological activity, and pharmacokinetic properties. They also improve patient acceptance and compliance while reducing drug toxicity.
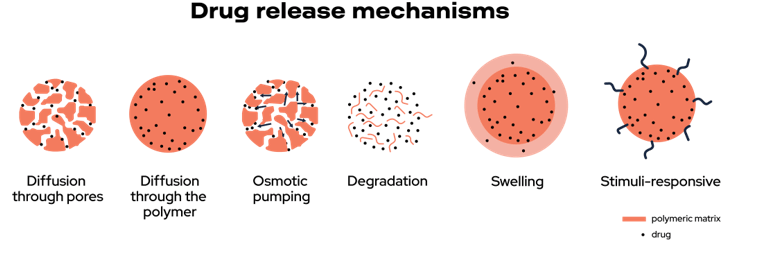
Main Release Mechanisms
Recently, more advanced drug delivery systems have been developed to provide more convenient, controlled, and selective release. Each drug delivery system has its own characteristics that determine the release rate and mechanism. These differences largely stem from physical, chemical, and morphological properties, which ultimately influence their affinity for various pharmaceutical substances. Studies have identified diffusion, chemical reaction, solvent activation, and stimulus control as the main release mechanisms.
Controlled Diffusion
One of the most common mechanisms is diffusion. The drug is released from a reservoir through a permeable polymer membrane. This method allows the release rate to be adjusted by controlling the thickness and permeability of the membrane. While it offers advantages like ease of manufacturing, one challenge is that the release rate decreases over time.
Chemical Activation
Chemically activated release occurs through a reaction involving a drug bound to a soluble polymer chain. While this method allows for high drug loading, it requires research into the cytotoxicity of the system. The erosion of biodegradable polymer matrices is another option, using aqueous or enzymatic hydrolysis.
Solvent Control
Solvent-controlled release mechanisms rely on the swelling of hydrophilic polymers or osmosis. In the first case, water absorption causes the polymer chains to relax, allowing drug diffusion. In the second, an osmotic device uses a semipermeable membrane that allows water to enter, forcing the drug out.
Stimuli-Responsive Systems
Lastly, stimuli-responsive systems are a promising area, as they react to internal or external triggers to release the drug. These systems enhance targeted drug delivery, reducing toxicity while increasing efficacy.
Current Drug Delivery Systems and Applications
In recent years, significant progress has been made in the development of drug delivery systems based on organic, inorganic, and hybrid nanoparticles as carriers for targeted therapies. The most recent systems have been formulated with improved properties, such as smaller particle size, enhanced permeability, solubility, efficacy, targeted localization, stability, reduced toxicity, and sustained release. This method of delivery can significantly boost the performance of therapeutic agents compared to conventional dosage forms.
Below are some of the different types of drug delivery systems:
- Liposomes
- Nanoparticles
- Dendrimers
- Transdermal systems
- Hydrogels
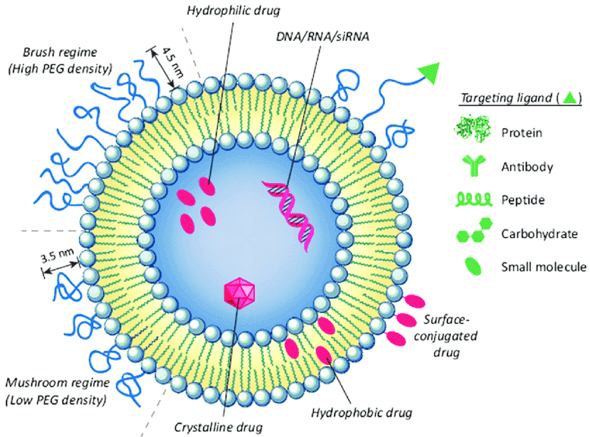
Liposomes
Liposomes are tiny synthetic, spherical vesicles made up of phospholipids and cholesterol.
Thanks to their small size (0.025 μm – 2.5 μm) and both hydrophilic and hydrophobic properties, they are used as carriers for drug delivery.
Their characteristics depend on lipid content, surface charge, size, and production techniques.
The choice of additives in the bilayer affects its tension or fluidity, as well as the charge. Liposomes form closed structures when phospholipids are hydrated in aqueous solutions.
Depending on the medicinal compound, they can carry water-soluble or lipid-soluble drugs within one or more bilayer membranes. The self-aggregation of polar lipids varies with molecular shape, temperature, and environmental conditions, which can lead to the formation of colloidal particles.
Liposomes have been used to deliver drugs such as paclitaxel, cyclosporine, cefepime, cabazitaxel, curcumin, and ibuprofen, enhancing their effectiveness and stability.
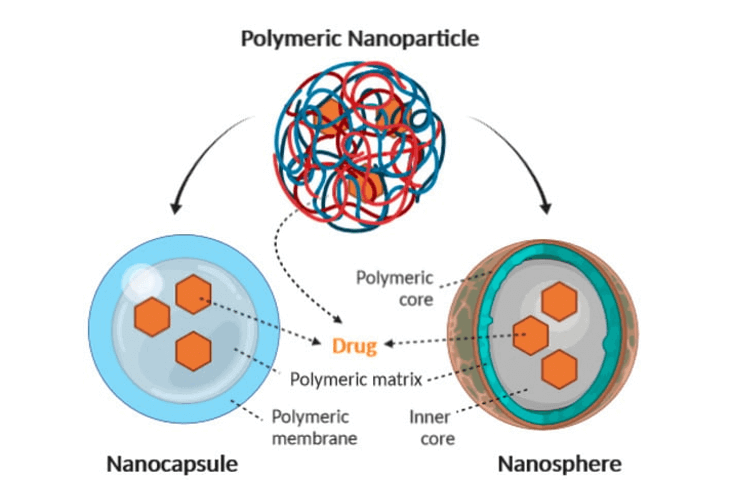
Nanoparticles
Nanoparticles are dispersions of particles with diameters ranging from 10 to 1000 nm. The nanoparticle matrix dissolves, entraps, encapsulates, or binds drugs. Depending on the preparation method, nanoparticles, nanocapsules, or nanospheres can be obtained.
Nanocapsules are matrix systems where the drug is physically and uniformly distributed, while nanospheres are matrix systems where the drug is contained in a cavity surrounded by a single polymer membrane.
Some advantages of nanoparticles include sustained and regulated drug release throughout transit and at the target site, matrix elements capable of controlled drug release, high drug-loading capacity, and the possibility of adding surface-targeting ligands or applying magnetic guidance for specific drug localization.
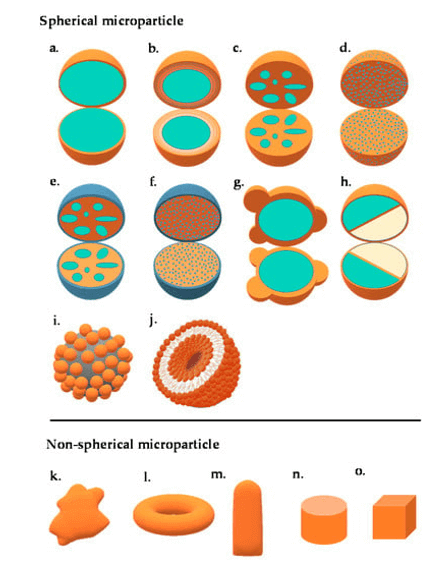
Microspheres
Microspheres are defined as monolithic spheres or therapeutic agents distributed throughout a matrix, either as a molecular dispersion of particles or structures composed of one or more miscible polymers in a continuous phase with molecular particles or macroscopic droplets.
These tiny, spherical particles range in size from 1 to 1000 μm. To prepare them, materials such as starches, gums, proteins, fats, and waxes, as well as biodegradable synthetic polymers and modified natural materials, are used.
Multiparticulate delivery methods enable uniform distribution throughout the gastrointestinal tract.
Unlike single-unit dosage forms, the polymer matrix does not dissolve, enhancing drug absorption and minimizing local irritation. Due to their small size, microspheres are widely dispersed throughout the gastrointestinal system, improving absorption and reducing the adverse effects of drugs that irritate the gastrointestinal mucosa.
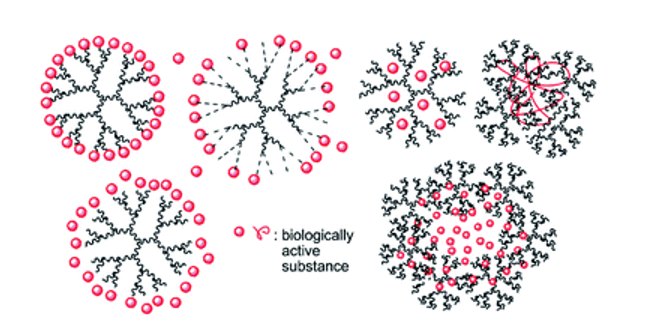
Dendrimers
Dendrimers have a dendritic structure with layers between each focal point known as generations or cascades.
They enable the delivery of chemotherapeutics, immunotherapeutics, and palliative treatments across the blood-brain barrier and can simultaneously deliver different chemicals with one or more mechanisms of action.
Dendrimers consist of three components: a central unit, branched dendrons, and surface ligands. Their advantages include direct delivery of medications to the affected area, selectivity in drug administration, increased permeability in solid tumors, and accumulation of macromolecules in tumors.
Drugs can be released in a controlled and continuous manner, designed to remain in the skin layers, thereby avoiding systemic circulation and bypassing the gastrointestinal tract to prevent fluctuations caused by gastric secretions. The drug loading capacity is relatively high.
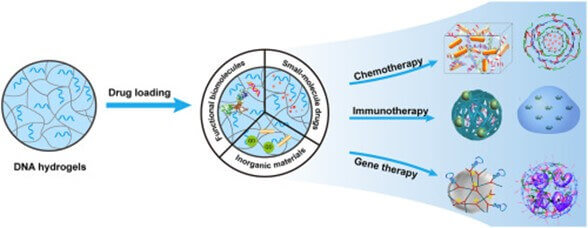
Hydrogels
Hydrogels are a three-dimensional polymeric network composed of natural and synthetic materials, known for their exceptional flexibility and high capacity to retain water.
They do not exist under physiological conditions but can maintain large volumes of water or biological fluids, exhibiting a gummy consistency similar to living tissues.
This makes them an excellent material for various applications, meeting requirements for functionality, reversibility, stability, and biocompatibility.
Hydrogels have elasticity comparable to that of real tissues, are biocompatible and biodegradable, allowing for injection and local application. Compared to traditional drug delivery methods, hydrogels provide a more sustained and prolonged effect, reduce side effects, enhance therapeutic compliance, and allow for targeted drug delivery to specific sites, such as the colon.
A Promising Future in Drug Delivery
Future Perspectives
The field of drug delivery has a bright and exciting future. As the current therapeutic landscape shifts from small molecules to biological products, drug delivery systems continue to evolve. Revolutionary technologies are expected to emerge that enhance the encapsulation and stability of biological products, allow for sustained release over extended periods, and facilitate effective delivery across complex physiological barriers.
Impact on Global Healthcare
Future drug delivery systems will also utilize materials that more efficiently and effectively target specific biological processes, be programmable, respond to biological signals, and integrate seamlessly to facilitate clinical translation. These future technologies will have a significant impact on global healthcare, not only by increasing the precision and effectiveness of therapies but also by making them more affordable and user-friendly.
Currently, many innovative treatments, while curative, are only accessible to those who can afford them. Reducing the healthcare costs of new pharmaceutical treatments, rather than waiting for lower-cost generic versions, will be crucial for the public, especially for individuals with limited access to medical resources. A series of innovations and combined efforts in low-cost, highly automated drug delivery technologies and manufacturing platforms will provide a significant boost toward this goal, thereby promoting health equity.
Recommended Reading on Advances in Biomaterials for Drug Delivery:
For those interested in the latest advancements in the use of biomaterials for drug delivery, particularly in cancer treatment and other therapies, we recommend reading the article “Biomaterials in Drug Delivery: Advancements in Cancer and Diverse Therapies,” published in the scientific journal MDPI. This article thoroughly reviews the progress made in the encapsulation and controlled release of drugs using biomaterials, providing a comprehensive overview of how these technologies can enhance therapeutic efficacy and reduce side effects.